Paul Burke of the Johns Hopkins University Applied Physics Laboratory decided to turn to a favorite tool of scientists everywhere: models.
Before we explore the model, examining the problem they are trying to solve is helpful. Electrolysis immerses an electrode in a liquid and uses an electrical current and subsequent chemical reaction to split atoms apart. So, for example, if you put an electrode in water, it would separate that water into hydrogen and oxygen.
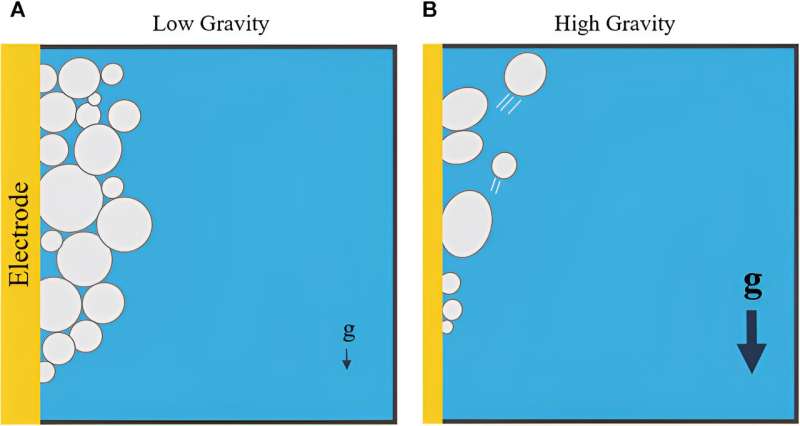
The problem comes from reduced gravity. As part of electrolysis, bubbles form on the surface of the electrode. On Earth, those bubbles typically detach and float to the surface, as the density difference between them and the remaining liquid forces them to.
However, in reduced gravity, the bubbles either take much longer to detach or don't do so at all. This creates a buffer layer along the electrode's length that decreases the electrolysis process's efficiency, sometimes stalling it out entirely. Electrolysis isn't the only fluidic process that has difficulty operating in reduced gravity environments—many ISS experiments also have trouble. This is partly due to a lack of complete understanding of how liquids operate in these environments—and that in itself is partly driven by a dearth of experimental data.
Which is where the modeling comes in. Dr. Burke and his colleagues use a technique known as Computational Fluid Dynamics to attempt to mimic the forces the fluids will undergo in a reduced gravity environment while also understanding bubble formation.
Electrolysis on Earth is typically done with water, but why stop there? The team used their CFD to model two other liquids that might be used in electrolyzers—molten salt (MSE) and molten regolith (MRE). The research is published in the journal Frontiers in Space Technologies.
Molten salt is used on Earth, but less commonly than regular water, and has successfully produced oxygen. However, molten regolith electrolysis is still somewhat of a novel use case and has yet to be thoroughly tested. MOXIE, the experiment that famously created oxygen on Mars in 2021, used the carbon dioxide in Mars' atmosphere and a solid-state electrode—neither representative of molten regolith.
Dr. Burke and his team found that, computationally, at least, MRE has the most challenging conditions in reduced gravity. It has also never been tested in any reduced gravity environment, so for now, these simulations are all engineers have to go on with if they are going to design a system.
There were a few key takeaways from the modeling, though. First, engineers should design horizontal electrodes into MRE systems, as the longer a bubble spreads across an electrode (i.e., as it goes "up" it), the longer it takes for that bubble to detach. In a horizontal configuration, the electrode has less surface area to attach to, making it more likely for the bubbles to detach and float to the surface.
Additionally, the amount of time bubbles remain attached to an electrode scales exponentially with decreasing gravity. That means bubbles on the moon will take longer to detach than those on Mars, which will take longer than those on Earth.
Consequently, electrolysis on the moon will be less efficient than that on Mars, which will again be less efficient than that on Earth, and mission planners will need to account for these discrepancies if they plan on getting something as mission-critical as oxygen from this process. The smoothness of the electrodes also seems to matter, with rougher electrodes more likely to hold onto their bubbles and, therefore, end up less efficient.
Other engineering solutions can overcome all these challenges, such as a vibratory mechanism on the electrode to shake the bubbles loose. However, it's a good idea to consider all the additional complications operations in a reduced gravity environment have before launching a mission. That's why modeling is so important, but humanity will ultimately have to experimentally test these systems, perhaps on the moon itself, if we plan to utilize its local resources to sustain our presence there.
More information: Paul A. Burke et al, Modeling electrolysis in reduced gravity: producing oxygen from in-situ resources at the moon and beyond, Frontiers in Space Technologies (2024). DOI: 10.3389/frspt.2024.1304579
Provided by Universe Today